As discussed previously, studying the microbiome has become of interest for many therapeutic applications. Thereby more and more projects include microbiome analysis. Talking about the microbiome often goes hand in hand with the word dysbiosis, especially when microbiome is being studied in pathological conditions. However, the notion of dysbiosis remains very obscure and variations from study to study on what it means may have an impact on research conclusions. We therefore found interesting to review the current knowledge and misconceptions about ‘dysbiosis’.
Recently, Hooks and O’Malley (2017) [1] have investigated the ways in which microbiome researchers discuss about dysbiosis by conducting an analysis of all uses of the word ‘dysbiosis’ (654 sources). According to this study, and some additional research, it has to be said that ‘no one seems able to define dysbiosis’. Authors tend to use abbreviated definitions (e.g. ‘changes in gut microbiota’). In some extreme cases, dysbiosis is not explained at all despite being the main subject of the article.
The gut microbiota is so variable (between individuals, pathologies, life stages …) that, in the current state of knowledge, dysbiosis should be considered at best as a relative term. In order to talk about dysbiosis, the scientific community should first better understand what eubiosis is. Although a universal healthy microbiota has not been defined yet, many bacteria have been associated to a healthy status. Yet, it remains premature to state that balance between any bacterial species is crucial for host health.
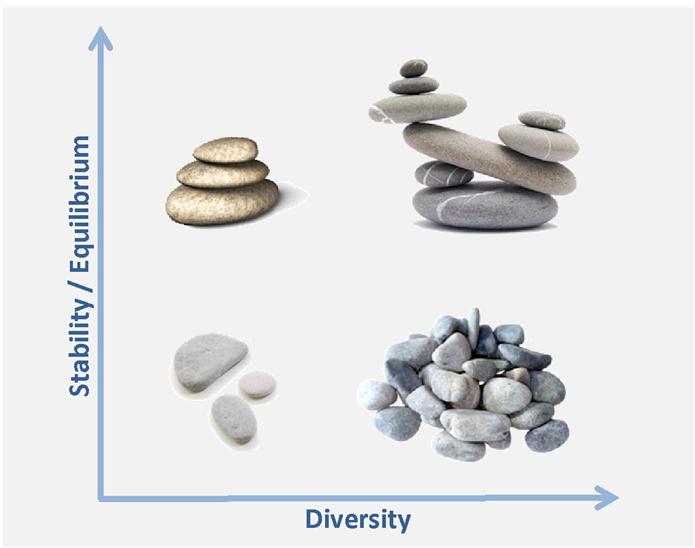
One hurdle is the way scientists analyze dysbiosis, focusing mostly on diversity (an estimation of how many species are there, and the evenness between these species). This is problematic for two reasons: i) this restrictive measure does not allow a great overview of the microbiome; ii) diversity does not mean stability, which seems to be more important for healthy microbiome characterization. Johnson and Burnets said [2], ‘we probably should diversify from diversity’.
Copyright: Biofortis Mérieux NutriSciences.
Indeed, there is another way to address the problem, which is to consider the metabolism potential rather than the composition. The basic method for that is to look at the gene count, and to consider that the more genes there are, the more metabolic functions there are.
The general idea is that stability, associated with healthy gut microbiome, is not based on taxonomy but rather on metabolic capabilities for which competent species are in competition.
On one hand, this point of view is consistent with the gut microbiome inter-individual variability: functions are achieved by different bacterial species depending on the selection pressure, inducing compositional modulation without functional modifications.
On the other end, the high gene count concept separates statistic from medical knowledge. Indeed, there are some cases where a low gene count is not associated with unhealthy microbiome: take a person with a very low diversified diet, his gut microbiome will have less metabolic functions, but that does not mean that he is unhealthy.
In conclusion, in the current state of knowledge it is difficult to say what is right or wrong, just remember that a microbiome composition change is not necessarily associated with a change in the functionality of this community.
One explication to why dysbiosis is so difficult to define could be that it follows the Anna Karenina principle according to Zanveld et al. [3]. Paralleling Tolstoy’s dictum that ‘all happy families look alike; each unhappy family is unhappy in its own way’, all healthy microbiome looks alike, and all dysbiotic microbiome could be dysbiotic in its own way. Such a broad phenomenon is difficult to encompass in a single definition.
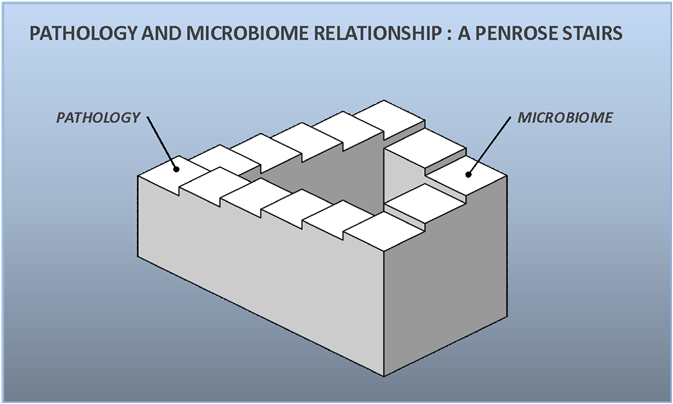
Trying to find the definition of dysbiosis looks actually like climbing Penrose stairs, all the blocks come together and follow each other to get you back to the starting point. For example, dysbiosis is often defined as an imbalance and an imbalance as the loss of homeostasis, but those two words are found together in many definitions, caught into a loop: imbalance as the lost homeostasis, homeostasis as the balance state.
Copyright: Biofortis Mérieux NutriSciences.
Finally, this misunderstanding of dysbiosis concept can even be counterproductive. According to Olesen and Alm (2016) [4]: ‘the balance concept […] is irrelevant to – and may even distract from – useful microbiome research’. As it is, any microbiome change, whether the cause or the effect of a pathological state could be called dysbiosis. Depending on how it is used, an observed correlation between dysbiosis and the disease could be merged with a causal link and this kind of confusion is of course a science injury.
As Olesen and Alm so rightly said, ’limiting the observation to the fact that healthy and ill people have different microbiomes is no longer a novel or useful observation’ [4]. The next step will be to try to understand the eubiosis concept and limits, for eventually defining a ‘dysbiosis threshold’, if there is one.
References
Hooks Katarzyna B. and O’Malley Maureen A. 2017. « Dysbiosis and Its Discontents ». mBio 8, no 5.Johnson Katerina VA and Burnet Philip W. J. 2016. « Microbiome: Should we diversify from diversity? » Gut Microbes 7, 6:455-58.Zaneveld Jesse R., McMinds Ryan, et Vega Thurber Rebecca. 2017. « Stress and Stability: Applying the Anna Karenina Principle to Animal Microbiomes ». 2017. Nature Microbiology 2, 9:17121.Olesen Scott W. and Alm Eric J. 2016.« Dysbiosis Is Not an Answer ». Nature Microbiology 1:16228.
Author: Lou Beuvin
Supervisor: Thomas Carton
Proofreading: Sidonie Lavergne
